
The half-life of U-238 is over 4.5 billion years the half-life of U-235 is over 700 million years. Table 1 depicts the total world uranium production in 1997.Īlthough the nucleus of the uranium atom is relatively stable, it is radioactive, and will remain that way for many years. Although uranium is found nearly everywhere on the earth, Canada leads the world in uranium production, mostly due to its heavy financial investment in uranium exploration, and to a few sizable deposits in the Saskatchewan territory. Uranium is a metal, and thus its acquisition is not unlike the mining of any other metallic ore. Most uranium deposits suitable for mining, however, contain an average of less than 1 percent uranium. Substantially greater average concentrations can be found in mineral deposits, as high as 10,000 ppm, or 10 percent. Uranium is found in most rock, in a concentration of two to four parts per million (ppm).
The enriched UF 6 is then converted into uranium dioxide (UO 2), and pressed into fuel pellets for use in the nuclear reactor. The UF 6 is next enriched, increasing the concentration of U-235 by separating out UF 6 made with U-238 atoms. After uranium is mined from geological mineral deposits, it is purified and converted into uranium hexafluoride (UF 6). But before uranium can be used in a nuclear reactor, it must undergo several processes. Nuclear power is now the only substantial use for uranium.
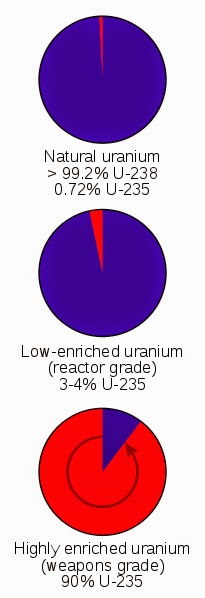
The isotope U-234 is actually a product of radioactive decay of U-238. A third isotope, U-234, also occurs naturally, but accounts for less than 0.01 percent of the total naturally occurring uranium. The isotope U-235, the second most abundant naturally occurring isotope, accounts for another 0.7 percent. The most commonly occurring natural isotope of uranium, U-238, accounts for approximately 99.3 percent of the world's uranium. Uranium (symbol: U atomic number: 92) is the heaviest element to occur naturally on Earth. Mixed oxide fuel, a combination of uranium and recovered plutonium, also has limited application in nuclear fuel, and will be briefly discussed.

These two nuclear fuels are discussed separately in order to explore their similarities and differences. Plutonium is created during the uranium fission cycle, and after being created will also fission, contributing heat to make steam in the nuclear power plant. Uranium is used as the primary source of nuclear energy in a nuclear reactor, although one-third to one-half of the power will be produced from plutonium before the power plant is refueled. A nuclear reactor creates steam with the heat produced from a controlled chain reaction of nuclear fission (the splitting of atoms). A fossil plant relies on the combustion of natural resources (coal, oil) to create steam. The fundamental difference is the source of heat to create the steam that turns the turbine-generator. A nuclear power plant generates electricity in a manner similar to a fossil fuel plant.


 0 kommentar(er)
0 kommentar(er)
